-
Pigments based on silica-coated gold nanorods: Synthesis, colouring strength, functionalisation, extrusion, thermal stability and colour evolution
C. Gautier, A. Cunningham, L. Si-Ahmed, G. Robert and T. Bürgi
Gold Bulletin, 43 (2) (2010), p94-104


unige:14683 | Article PDF
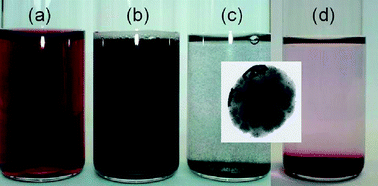
The intense plasmon absorption bands of gold nanorods (GNRs) with peak extinction coefficients up to 6.4 x 109 M-1 cm-1 as well as their expected high stability make GNRs promising candidates for the colouration of bulk materials. The comparison of the integrated absorption in the visible region of GNRs with those of commercial organic pigments shows that the colouring strength of GNRs is 4 to 8 times higher. In order to improve their stability, GNRs were encapsulated in a silica shell of around 15 nm thickness using an optimized Stöber method. The silica surface was modified with octadecylsilane to enable their dispersion in non-polar media. Different plastics were successfully coloured with a tiny quantity of bare and functionalised GNRs@SiO2. These rods were homogeneously dispersed using extrusion. The shape of the rods was effectively stabilised by the silica shell at high temperature during the extrusion process. Surprisingly, a slight modification of the rods colour was observed due to a decrease of the refractive index in the mesoporous silica shell. However, this effect is greatly limited after the functionalisation.